

Date
February 23, 2023
In the spirit of harmonisation, Label2Enable has been collaborating with the Finnish Coordinating Center for Health Technology Assessment (FinCCHTA) and the University of Oulu to see how CEN-ISO/TS 82304-2 and the Finnish Digi-HTA framework align.
FinCCHTA and the University of Oulu developed the Digi-HTA method in the period 2018-2019. The method can be applied to assess digital products and services, such as mHealth, artificial intelligence and robotics, for social and health care and well-being and their suitability for use by customers and employees in Finland.
With CEN-ISO/TS 82304-2 being an international technical specification, in 2022 FinCCHTA carried out a comparison of the two approaches in order to inform Digi-HTA’s further development. The comparison report can be downloaded from FinCCHTA's website.
While CEN-ISO/TS 82304-2 focuses on health and wellness apps defined as software applications that can be executed (run) on a computing platform intended to be used specifically for managing, maintaining, or improving health of individual persons, or the delivery of care, Digi-HTA also includes assessment of hardware devices with embedded software integrations in their operating environment.
Similar to CEN-ISO/TS 82304-2, Digi-HTA includes several assessment aspects. CEN-ISO/TS 82304-2’s quality aspects include Healthy and safe (comprising health requirements, health risks, ethics, health benefit and societal benefit), Easy to use (accessibility, usability), Secure data (privacy, security), and Robust build (technical robustness, interoperability). Digi-HTA differentiates between effectiveness, costs, safety, data protection and security, usability, and accessibility. The evidence needed for the assessment is also mostly provided by the owner of the digital solution / technology, which Digi-HTA typically supplements with a literature review and expert reviews.
The outcome of the Digi-HTA assessment is a traffic light model comparable in function to the CEN-ISO/TS 82304-2 label. The Digi-HTA model gives a recommendation which is valid for three years and can undergo reassessment upon the request of the product / service provider in case of significant product / service changes. The Label2Enable project is in the process of co-creating an ISO 17065 certification scheme for CEN-ISO/TS 82304-2. This scheme aligns with EU level legislation and EU values and includes how to qualify as an app assessor, assessment methods employed, what is sufficient evidence, when to reassess an app, how to do surveillance and when to withdraw a label. This certification scheme evolves over the course of 2023 in testing it with 24 app manufacturers and 6 app assessment organisations from 6 different countries. The group of 6 app assessment organisations includes 2 notified bodies, which enables us to jointly find out how to avoid duplicating the work of notified bodies in case a health app is a Class IIa, IIb or III medical device.
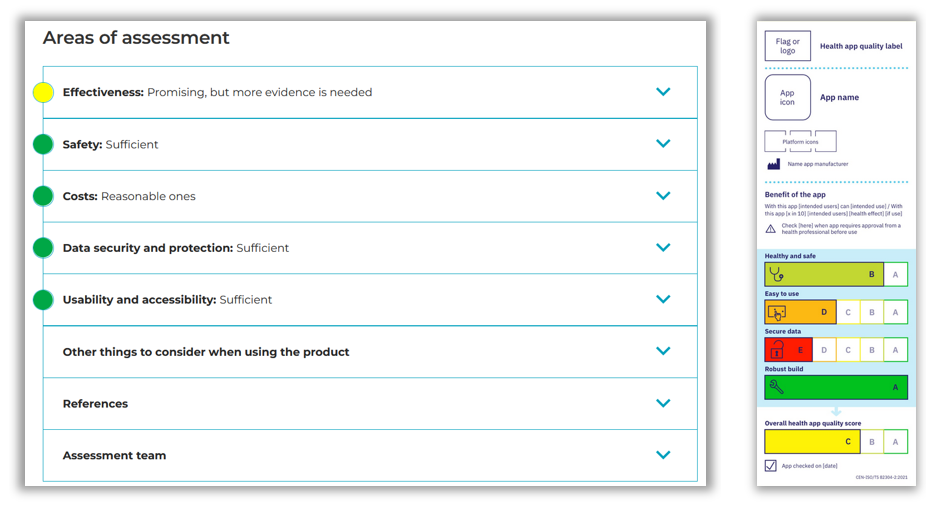
Both Digi-HTA (left) and the CEN-ISO/TS 82304-2 label (right) use traffic light colour coding
While Label2Enable works towards decentralised assessments with accredited app assessment organisations, measuring consistency in assessments, efficiency and to which extent documentation is found to be self-explanatory, the Digi-HTA assessments are currently carried out centrally by FinCCHTA and the University of Oulu.
The comparison has been valuable for FinCCHTA because it informs the Digi-HTA assessment criteria and their further development, which strive to provide a national basis for assessment while still offering high complementarity with international/EU-level assessments. FinCCHTA is committed to continuing to align internationally and is also involved in the European Taskforce for Harmonised Evaluation of Digital Medical Devices, as is the Label2Enable coordinator. Label2Enable seeks to further evolve the certification scheme to provide manufacturers with the harmonised basis in health app assessment, also referred to as the Digital Single Market, that prevents them from having to undertake a similar assessment 27 or more times and enables national, regional, and local authorities the opportunity to focus on the potentially remaining context specific requirements.
For more information about the Digi-HTA model, visit www.digi-hta.fi